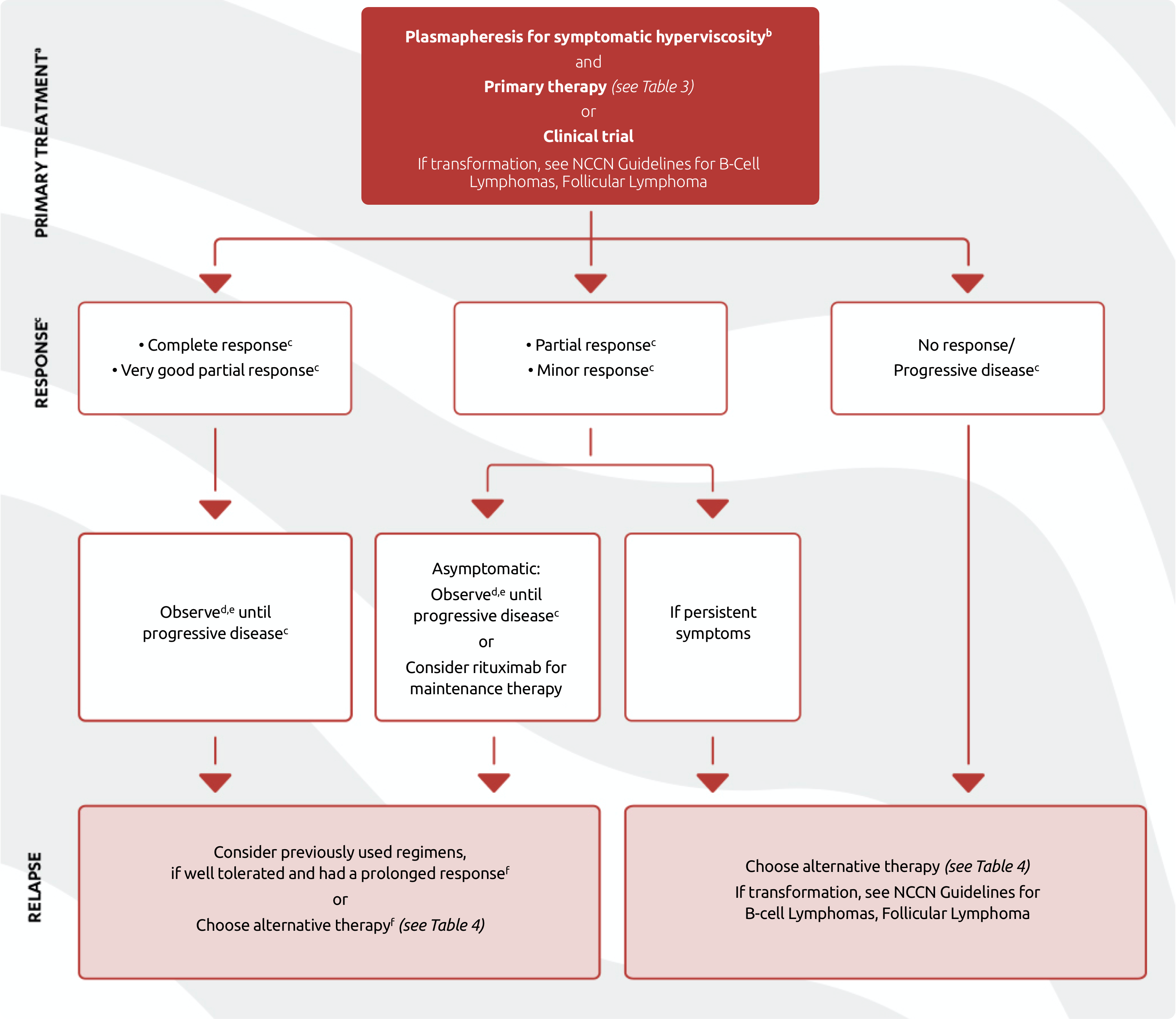
TREATMENT PATHWAYS IN SYMPTOMATIC WM
The choice of therapeutic agent is guided by disease presentation and patient-related factors including age, comorbidities and complications requiring immediate treatment response.1
The NCCN Guidelines for Waldenström Macroglobulinemia/Lymphoplasmatic Lymphoma outline a recommended treatment pathway for primary treatment, response and relapse of symptomatic WM (Figure 3).2
- Intent of therapy should be based on palliation of symptoms, not necessarily levels of IgM unless the patient is exhibiting evidence of symptomatic hyperviscosity.
- Plasmapheresis should be performed for patients with symptomatic hyperviscosity, and before treatment with rituximab-containing regimen in patients with IgM ≥4000 mg/dL. IgM should be monitored closely in these patients thereafter and plasmapheresis should be considered again if symptomatic hyperviscosity recurs or if IgM is ≥4000 mg/dL while on rituximab-containing therapy. RBC transfusion, if indicated, should be done after plasmapheresis to prevent added hyperviscosity load.
- Complete Response (requires two consecutive assessments made at any time before the institution of any new therapy): IgM in normal range and disappearance of monoclonal protein by immunofixation; no histologic evidence of bone marrow involvement, and resolution of any adenopathy/organomegaly (if present at baseline), along with no signs or symptoms attributable to WM. Reconfirmation of the CR status is required by repeat immunofixation studies. Very Good Partial Response: A ≥90% reduction of serum IgM and decrease in adenopathy/organomegaly (if present at baseline) on physical examination or on CT scan. No new symptoms or signs of active disease. Partial Response: A ≥50% reduction of serum IgM and decrease in adenopathy/organomegaly (if present at baseline) on physical examination or on CT scan. No new symptoms or signs of active disease. Minor Response: A ≥25% but <50% reduction of serum IgM. No new symptoms or signs of active disease. Stable Disease: A <25% reduction and <25% increase of serum IgM without progression of adenopathy/organomegaly, cytopenias, or clinically significant symptoms due to disease and/or signs of WM. Progressive Disease (requires two consecutive assessments made at any time before the institution of any new therapy): A ≥25% increase in serum IgM by protein confirmed by a second measurement or progression of clinically significant findings due to disease (i.e., anemia, thrombocytopenia, leukopenia, bulky adenopathy/organomegaly) or symptoms (unexplained recurrent fever ≥38.4ºC, drenching night sweats, ≥10% body weight loss, or hyperviscosity, neuropathy, symptomatic cryoglobulinemia, or amyloidosis) attributable to WM.
- See NCCN Guidelines for survivorship.
- CBC, complete metabolic panel, and IgM every 3 months for 2 years, then every 4–6 months for additional 3 years, then every 6–12 months. Progression based on IgM levels alone, without symptoms, should not be reason to retreat.
- Caution should be used when re-treating with myelosuppressive regimens due to cumulative toxicities.
Adapted with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Waldenström Macroglobulinemia/Lymphoplasmatic Lymphoma V.2.2022 – December 7, 2021 © 2022 National Comprehensive Cancer Network, Inc. All rights reserved. The NCCN Guidelines® and illustrations herein may not be reproduced in any form for any purpose without the express written permission of the NCCN. To view the most recent and complete version of the NCCN Guidelines, go online to NCCN.org. The NCCN Guidelines are a work in progress that may be refined as often as new significant data becomes available. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
(Order of regimens is alphabetical and does not indicate preference)
- Bendamustine/rituximab
- Bortezomib/dexamethasone/rituximabb
- Ibrutinib ± rituximab (category 1)
- Rituximab/cyclophosphamide/dexamethasone
- Zanubrutinib (category 1)
-
- Bendamustine
- Bortezomib ± rituximabb
- Bortezomib/dexamethasone
- Carfilzomib/rituximab/dexamethasone
- Cladribine ± rituximabc
-
- Fludarabine ± rituximabc
- Fludarabine/cyclophosphamide/
rituximabc - Ixazomib/rituximab/dexamethasone
- Rituximab
- Rituximab /cyclophosphamide/prednisone
- See General Considerations for Systemic Therapy for WM/LPL (WM/LPL-B 1 of 4). NCCN Guidelines for Older Adult Oncology.
- Consider for patients presenting with symptomatic hyperviscosity, or in whom rapid IgM reduction is required.
- May be associated with disease transformation and/or development of MDS/AML in patients with Waldenström Macroglobulinemia.
Adapted with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Waldenström Macroglobulinemia/Lymphoplasmatic Lymphoma V.2.2022 – December 7, 2021 © 2022 National Comprehensive Cancer Network, Inc. All rights reserved. The NCCN Guidelines® and illustrations herein may not be reproduced in any form for any purpose without the express written permission of the NCCN. To view the most recent and complete version of the NCCN Guidelines, go online to NCCN.org. The NCCN Guidelines are a work in progress that may be refined as often as new significant data becomes available. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.

The NCCN strongly encourages treatment in the context of a clinical trial when possible, for all patients, either in first line or subsequent lines of therapy.2
The NCCN Guidelines for Waldenström Macroglobulinemia/Lymphoplasmatic Lymphoma outline therapy for previously treated WM (Table 4).2
(Order of regimens is alphabetical and does not indicate preference)
- Bendamustine/rituximab
- Bortezomib/dexamethasone/rituximabb
- Ibrutinib ± rituximab (category 1)
- Rituximab/cyclophosphamide/dexamethasone
- Zanubrutinib (category 1)
- Everolimus
- Ofatumumab (for rituximab-intolerant individuals)d
- Acalabrutinib
- Bendamustine
- Bortezomib ± rituximabb
- Bortezomib/dexamethasone
- Cladribine ± rituximabc
- Cyclophosphamide/doxorubicin/vincristine/
prednisone/rituximab - Fludarabine ± rituximabc
- Fludarabine/cyclophosphamide/rituximabc
- Rituximab
- Rituximab/cyclophosphamide/prednisone
- Venetoclax
- In selected cases hematopoietic cell transplantation may be appropriate with either:
- Allogeneic hematopoietic cell transplant (ablative or non‑ablative)e
- Autologous hematopoietic cell transplant
- See General Considerations for Systemic Therapy for WM/LPL (WM/LPL-B 1 of 4).
- Consider for patients presenting with symptomatic hyperviscosity, or in whom rapid IgM reduction is required.
- May be associated with disease transformation and/or development of MDS/AML in patients with Waldenström Macroglobulinemia.
- Ofatumumab may be used for rituximab-intolerant individuals as a single agent or in combination therapy anywhere that rituximab is given. While ofatumumab is no longer commercially available, it may be obtained for clinical use.
- Should ideally be undertaken in the context of a clinical trial.
Adapted with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Waldenström Macroglobulinemia/Lymphoplasmatic Lymphoma V.2.2022 – December 7, 2021 © 2022 National Comprehensive Cancer Network, Inc. All rights reserved. The NCCN Guidelines® and illustrations herein may not be reproduced in any form for any purpose without the express written permission of the NCCN. To view the most recent and complete version of the NCCN Guidelines, go online to NCCN.org. The NCCN Guidelines are a work in progress that may be refined as often as new significant data becomes available. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
References:
- Leukemia & Lymphoma Society. Waldenström macroglobulinemia facts. Available at: https://www.llscanada.org/sites/default/files/file_assets/FS20_Waldenstrom_M%20FINAL%206.15.pdf. Accessed August 24, 2021.
- Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Waldenström Macroglobulinemia/Lymphoplasmatic Lymphoma V.2.2022 – December 7, 2021 © National Comprehensive Cancer Network, Inc. 2022. All rights reserved. Accessed March 4, 2022. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.