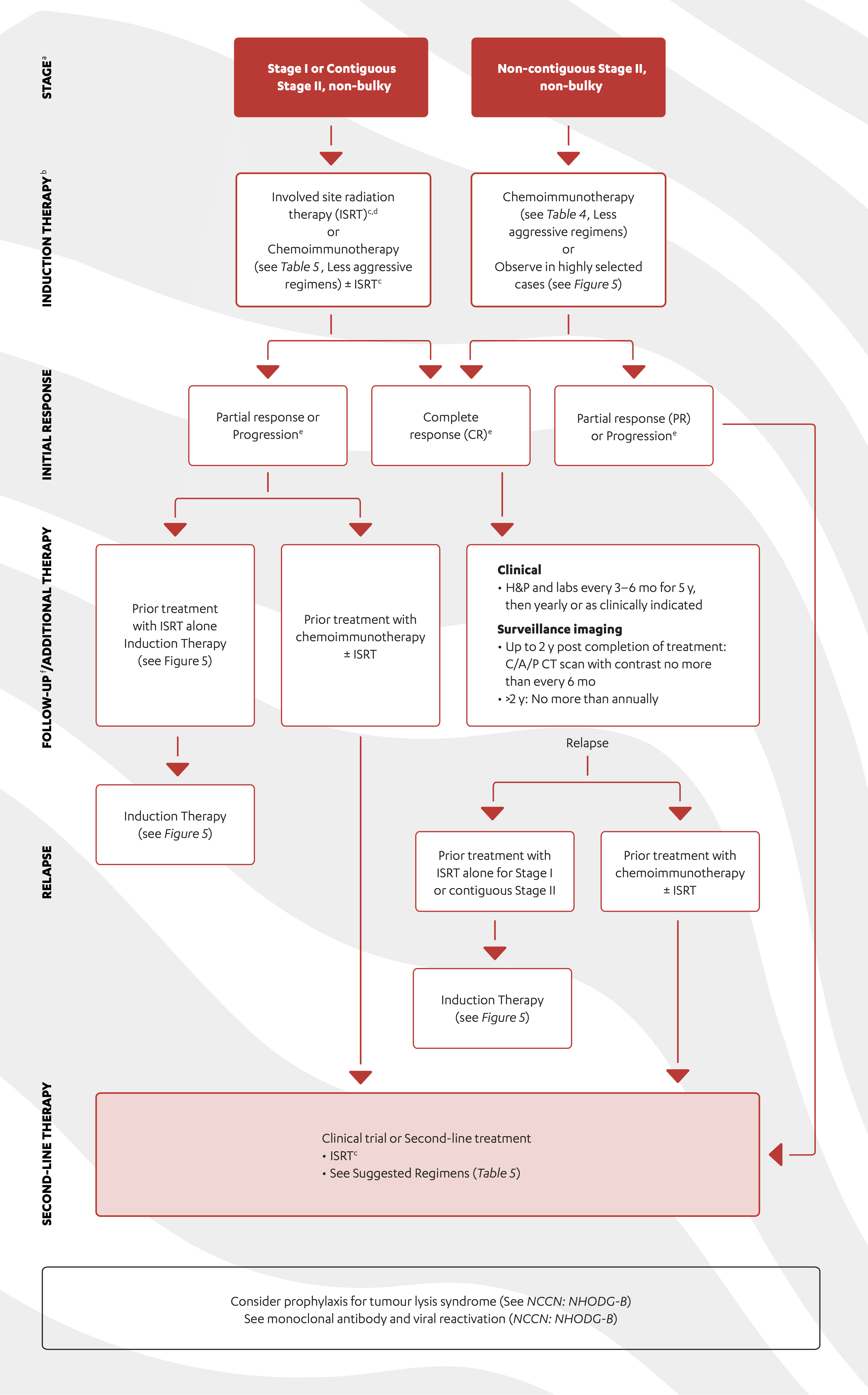
SYMPTOMATIC MCL: FIRST-LINE TREATMENT
The first-line choice of therapeutic agent will be guided by clinical risk factors, symptoms and patient characteristics.1,2
Suggested first-line treatment choices1–3
- For younger, fit, patients first-line treatment should be based on a dose-intensified chemoimmunotherapy regimen, with the option of autologous stem cell rescue (ASCR). This should be followed with rituximab maintenance therapy1,2
- Older, fit patients should be offered conventional chemoimmunotherapy, followed by rituximab maintenance1–3
- In frail patients, a less-intense chemoimmunotherapy regimen may be considered1,2
The NCCN Guidelines® for B-Cell Lymphomas outline recommended treatment pathways for induction therapy, response, relapse and second-line treatment of MCL.1
- Localized presentation is extremely rare.
- Early referral for high-dose therapy with stem cell rescue is advisable for planning purposes.
- See NCCN: Principles of Radiation Therapy (NCCN: NHODG-D).
- Leitch HA, Gascoyne RD, Chhanabhai M, et al. Limited-stage mantle-cell lymphoma. Ann Oncol 2003;14:1555-1561.
- See NCCN: Lugano Response Criteria for Non-Hodgkin Lymphoma (NCCN: NHODG-C).
- Follow-up includes diagnostic tests and imaging using the same modalities performed during workup as clinically indicated.
Adapted with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for B-Cell Lymphomas V.1.2022 – March 2, 2022. © 2022 National Comprehensive Cancer Network, Inc. All rights reserved. The NCCN Guidelines® and illustrations herein may not be reproduced in any form for any purpose without the express written permission of the NCCN. To view the most recent and complete version of the NCCN Guidelines, go online to NCCN.org. The NCCN Guidelines are a work in progress that may be refined as often as new significant data becomes available. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
FIGURE 5. STAGE II BULKY, III, IV
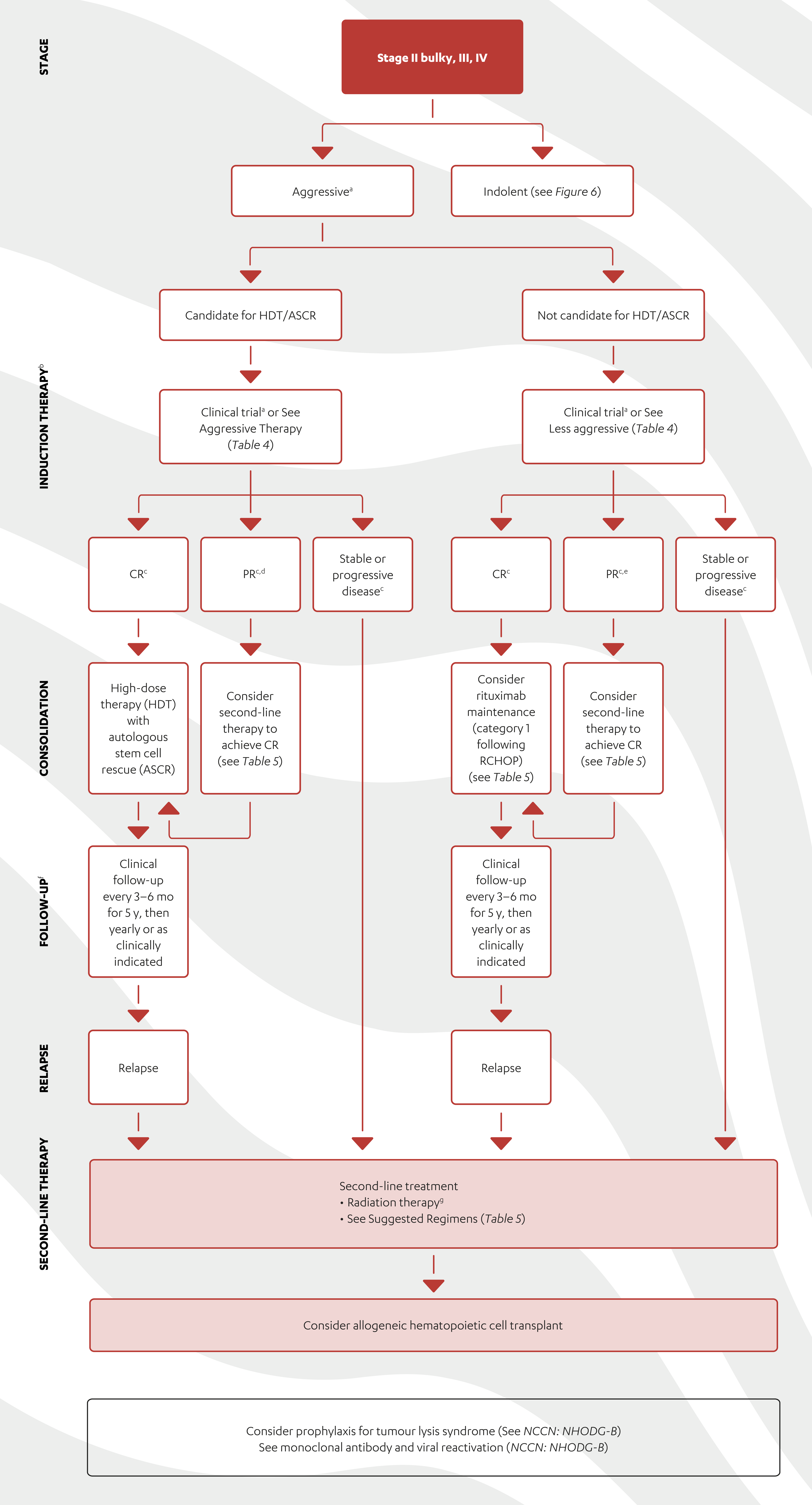
- TP53 mutation has been associated with poor prognosis in patients treated with conventional therapy, including transplant. Clinical trial is strongly suggested for these patients.
- Early referral for high-dose therapy with stem cell rescue is advisable for planning purposes.
- See NCCN: Lugano Response Criteria for Non-Hodgkin Lymphoma (NCCN: NHODG-C).
- Patients who have achieved near CR can proceed to HDT/ASCR. Patients who have achieved minimal PR with substantial disease should be treated as having stable, refractory disease. Patients who have achieved a very good PR may be treated with additional therapy to achieve CR with the goal of proceeding to HDT/ASCR.
- Patients who have achieved a very good PR or better can be observed or consider rituximab maintenance. Patients who have achieved minimal PR with substantial disease should be treated as having stable, refractory disease.
- Follow-up includes diagnostic tests and imaging using the same modalities performed during workup as clinically indicated.
- See NCCN: Principles of Radiation Therapy (NCCN: HODG-D).
Adapted with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for B-Cell Lymphomas V.1.2022 – March 2, 2022. © 2022 National Comprehensive Cancer Network, Inc. All rights reserved. The NCCN Guidelines® and illustrations herein may not be reproduced in any form for any purpose without the express written permission of the NCCN. To view the most recent and complete version of the NCCN Guidelines, go online to NCCN.org. The NCCN Guidelines are a work in progress that may be refined as often as new significant data becomes available. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
FIGURE 6. INDOLENT
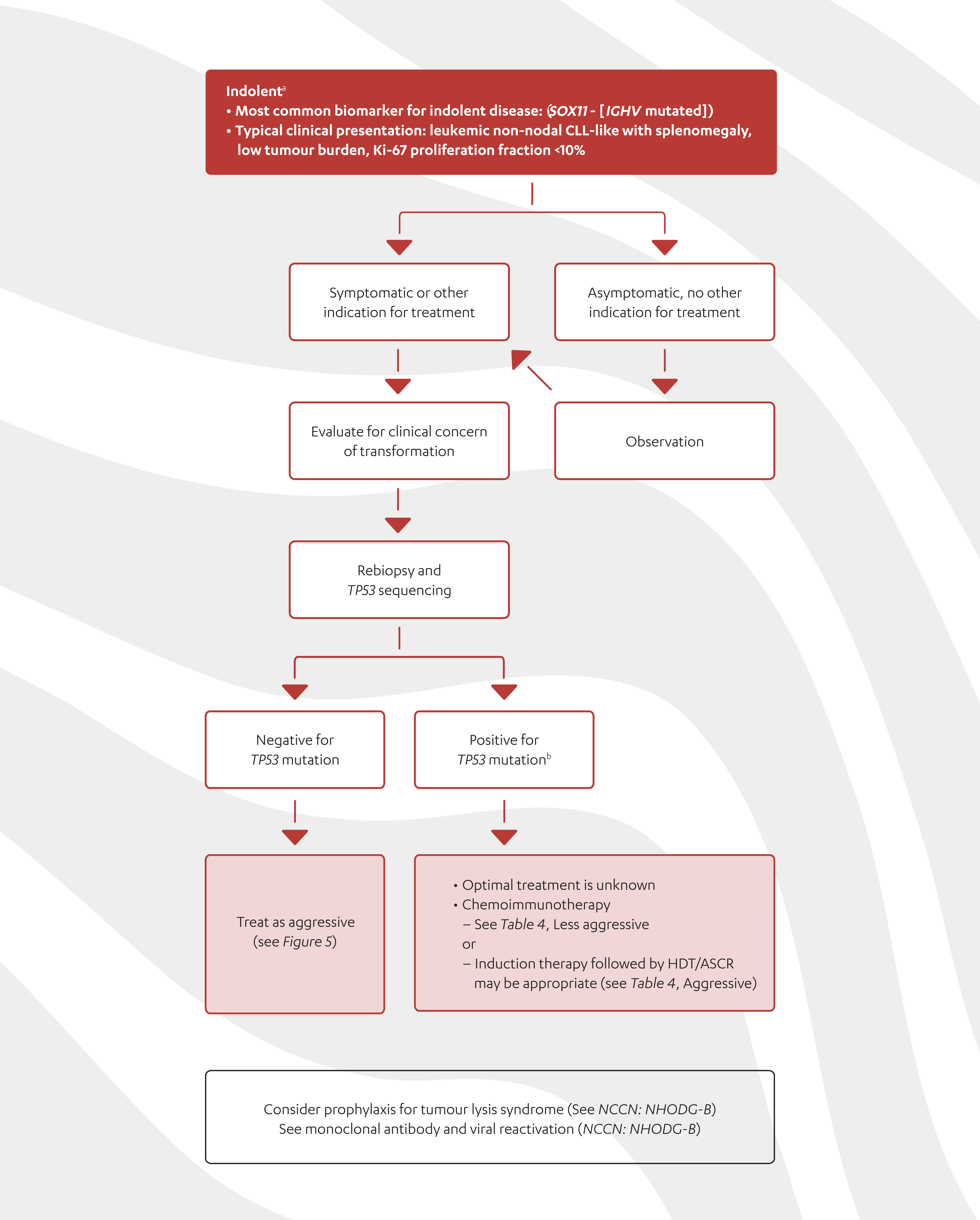
- The description represents the most common indolent presentation; however, there are some patients with GI or blood/bone marrow involvement only, which may express SOX11 and have an indolent course.
- TP53 mutation has been associated with poor prognosis in patients treated with conventional therapy, including transplant. Clinical trial is strongly suggested for these patients.
Adapted with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for B-Cell Lymphomas V.1.2022 – March 2, 2022. © 2022 National Comprehensive Cancer Network, Inc. All rights reserved. The NCCN Guidelines® and illustrations herein may not be reproduced in any form for any purpose without the express written permission of the NCCN. To view the most recent and complete version of the NCCN Guidelines, go online to NCCN.org. The NCCN Guidelines are a work in progress that may be refined as often as new significant data becomes available. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
(Some of the monotherapies and/or combination therapies listed below may be off-label in Canada. Refer to the respective Product Monographs for complete information.)
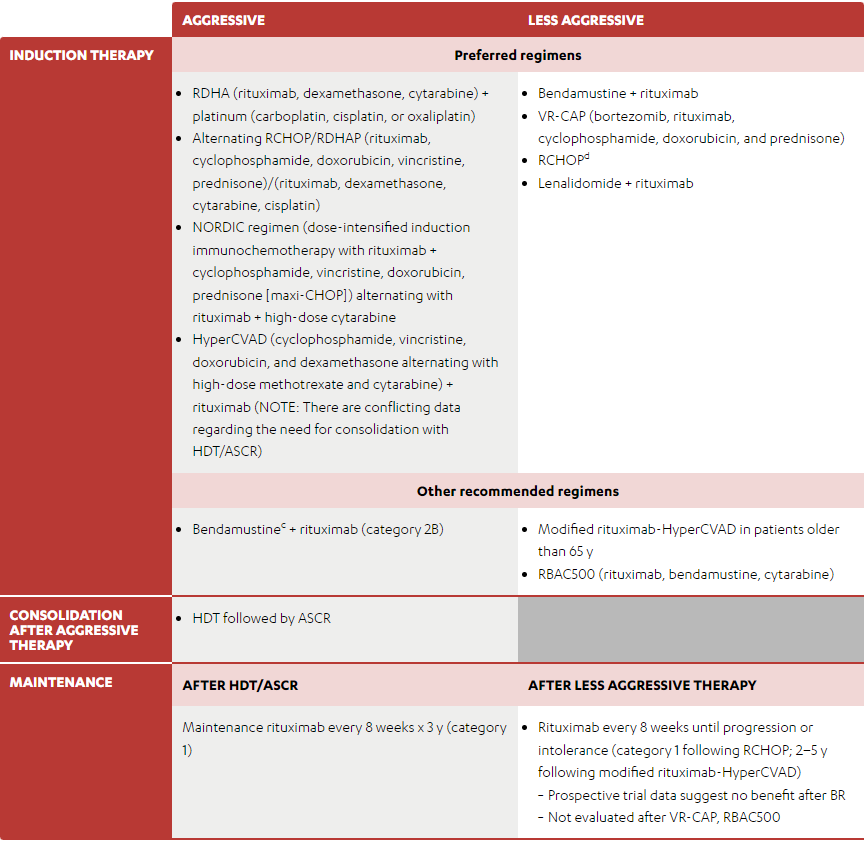
- RDHA (rituximab, dexamethasone, cytarabine) + platinum (carboplatin, cisplatin, or oxaliplatin)
- Alternating RCHOP/RDHAP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone)/(rituximab, dexamethasone, cytarabine, cisplatin)
- NORDIC regimen (dose-intensified induction immunochemotherapy with rituximab + cyclophosphamide, vincristine, doxorubicin, prednisone [maxi-CHOP]) alternating with rituximab + high-dose cytarabine
- HyperCVAD (cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with high-dose methotrexate and cytarabine) + rituximab (NOTE: There are conflicting data regarding the need for consolidation with HDT/ASCR)
- Bendamustine + rituximab
- VR-CAP (bortezomib, rituximab, cyclophosphamide, doxorubicin, and prednisone)
- RCHOPd
- Lenalidomide + rituximab
- Bendamustinec + rituximab (category 2B)
- Modified rituximab-HyperCVAD in patients older than 65 y
- RBAC500 (rituximab, bendamustine, cytarabine)
- HDT followed by ASCR
-
Rituximab every 8 weeks until progression or intolerance (category 1 following RCHOP; 2–5 y following modified rituximab-HyperCVAD)
- Prospective trial data suggest no benefit after BR
- Not evaluated after VR-CAP, RBAC500
- See references for regimens NCCN: MANT-A 3 of 4 and NCCN: MANT-A 4 of 4.
- Rituximab and hyaluronidase human injection for subcutaneous use may be substituted for rituximab after patients have received the first full dose of rituximab by intravenous infusion. This substitution cannot be made for rituximab used in combination with ibritumomab tiuxetan.
- In patients intended to receive HDT/ASCR, bendamustine should be used with caution as there are conflicting data regarding ability to collect peripheral progenitor cell collection.
- There is a randomized trial that demonstrated that RCHOP was not superior to CHOP.
Adapted with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for B-Cell Lymphomas V.1.2022 – March 2, 2022. © 2022 National Comprehensive Cancer Network, Inc. All rights reserved. The NCCN Guidelines® and illustrations herein may not be reproduced in any form for any purpose without the express written permission of the NCCN. To view the most recent and complete version of the NCCN Guidelines, go online to NCCN.org. The NCCN Guidelines are a work in progress that may be refined as often as new significant data becomes available. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
TABLE 5. SUGGESTED TREATMENT REGIMENS: SECOND-LINE THERAPYa,b
(Some of the monotherapies and/or combination therapies listed below may be off-label in Canada. Refer to the respective Product Monographs for complete information.)
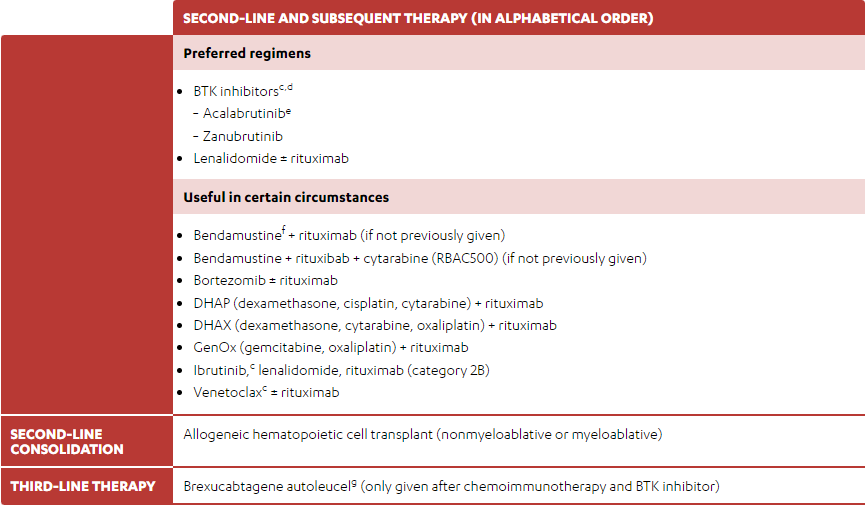
-
BTK inhibitorsc,d
- Acalabrutinibe
- Zanubrutinib
- Lenalidomide ± rituximab
- Bendamustinef + rituximab (if not previously given)
- Bendamustine + rituxibab + cytarabine (RBAC500) (if not previously given)
- Bortezomib ± rituximab
- DHAP (dexamethasone, cisplatin, cytarabine) + rituximab
- DHAX (dexamethasone, cytarabine, oxaliplatin) + rituximab
- GenOx (gemcitabine, oxaliplatin) + rituximab
- Ibrutinib,c lenalidomide, rituximab (category 2B)
- Venetoclaxc ± rituximab
Please refer to the NCCN Guidelines for complete information
- See references for regimens NCCN: MANT-A 3 of 4 and NCCN: MANT-A 4 of 4.
- Rituximab and hyaluronidase human injection for subcutaneous use may be substituted for rituximab after patients have received the first full dose of rituximab by intravenous infusion. This substitution cannot be made for rituximab used in combination with ibritumomab tiuxetan.
- See NCCN: Special Considerations for Use of Small-Molecule Inhibitors (NCCN: NHODG-E)
- Acalabrutinib and zanubrutinib have not been shown to be effective for ibrutinib-refractory mantle cell lymphoma with BTK C481S mutations. Patients with ibrutinib intolerance have been successfully treated with acalabrutinib or zanubrutinib without recurrence of symptoms.
- The phase 2 ACE-LY-004 study excluded patients treated with Bruton's tyrosine kinase (BTK) or BCL-2 inhibitor and concomitant warfarin or equivalent vitamin K antagonists.
- In patients intended to receive HDT/ASCR, bendamustine should be used with caution as there are conflicting data regarding ability to collect peripheral progenitor cell collection.
- See NCCN: Guidance for the Treatment of Patients with Chimeric Antigen Receptor (CAR) T-Cell Therapy (NHODG-F).
Adapted with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for B-Cell Lymphomas V.1.2022 – March 2, 2022. © 2022 National Comprehensive Cancer Network, Inc. All rights reserved. The NCCN Guidelines® and illustrations herein may not be reproduced in any form for any purpose without the express written permission of the NCCN. To view the most recent and complete version of the NCCN Guidelines, go online to NCCN.org. The NCCN Guidelines are a work in progress that may be refined as often as new significant data becomes available. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
References:
- Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for B-Cell Lymphomas V.1.2022 – March 2, 2022. © National Comprehensive Cancer Network, Inc. 2022. All rights reserved. Accessed March 4, 2022. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
- Leukemia & Lymphoma Society. Mantle cell lymphoma facts. Available at: https://www.llscanada.org/sites/default/files/National/CANADA/Pdf/InfoBooklets/FS4_MCL_2018_FINAL.pdf. Accessed August 24, 2021.
- Jain P and Wang M. Mantle cell lymphoma: 2019 update on the diagnosis, pathogenesis, prognostication, and management. Am J Hematol 2019;94:710–25.